Folliculogenesis In Vivo™ – edit 2023e
Right-click the image for better legibility – open it in new tab. Link to the cited G.D. Hodgen 1982 paper: The dominant ovarian follicle
First, a sketch of basic reproductive endocrinology of the mature follicle
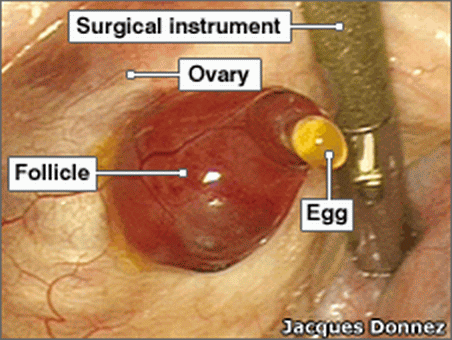
Ovarian follicles are tiny fluid-filled sacs containing immature egg cells that are periodically initiated to grow and develop, culminating in ovulation. This is how it goes in the process called folliculogenesis or rather in its final stage involving the mature (“dominant, Graafian“) follicle:
The peptide called follicle-stimulating hormone (FSH), which is released by the pituitary gland of the brain, primarily functions to induce proliferation of the follicular granulosa cells in one of the two ovaries and to stimulate an aromatase enzyme for estrogen synthesis.
The other pituitary peptide, luteinizing hormone (LH), then stimulates the transformation of estrogen-secreting stromal cells in the selected ovary into progesterone-secreting cells, and promotes ovulation. (The LH alone is tested for in the urine by the so-called OPKs, Ovulation Prediction Kits.)
The predominant ovarian hormones, which exert peripheral, central and intra-ovarian effects, are the so-called sex steroids – estrogen or estradiol (E2) and progesterone (P4). They are called sex hormones because, when they were discovered more than a century ago, people did not know their ubiquitous presence and activities all over the body.
There are also other steroids at play in the ovarian and hypothalamic-pituitary events, and there are also other peptidic reproductive hormones known as non-steroidal ovarian factors (e.g., inhibin, oocyte maturation inhibitor, gonadotropin surge-inhibiting factor, and certain growth factors).
Key take-home message:
Synchronized brain-ovary orchestration is the absolute requirement
The properly orchestrated actions of all the variously secreted substances are known to be necessary for the functioning of the menstrual cycle.
The endometrium is the glandular mucous membrane lining the uterus (or womb), and the cervix uteri is the glandular “neck of the womb”; they are very sensitive detectors of the hormonal signals and of their orchestration, that is the timing of the brain-ovary hormonal signals with respect to each other.
Said proper orchestration is the absolute prerequisite of properly working female reproductive mechanism, i.e., folliculogenesis. The Ovulona sensor monitors folliculogenesis.
Disclosed below is the following bioZhena’s working principle: Folliculogenesis is not merely a process involving hormonal signals – it’s a process of integration of all neuroendocrinological inputs, which the cervix monitors. And the Ovulona monitors the cervix.
Explanation of Folliculogenesis With Pictorial Summary
High-level definition
The periodically recurring development of ovarian follicles, in preparation for the periodically recurring ovulation, is called folliculogenesis. The process of folliculogenesis is the essence of ovarian function, and its dynamics is an absolute need-to-know for fertility status determination.
Folliculogenesis involves four basic conditions in which the many follicles – present from birth in the two ovaries – can be found. The four conditions are: resting, growing, atretic, or ready to ovulate [A.L. Goodman and G.D. Hodgen, in R.O. Greep, editor: Recent progress in hormone research, Academic Press, 1983]. Most of the follicles remain resting but, periodically in every menstrual cycle, a group of follicles are “recruited” to grow. Only one of these will mature and will normally ovulate, with the rest of the group succumbing to atresia (death).
It is well established that women and other primate females produce a single fertilizable egg approximately every four weeks, but that the actual duration of this circamensual (or approximately one-month) period is not a constant. Rather, the period ranges from about three weeks to about five weeks.
Therein lies the need for and the challenge of reliable monitoring of the menstrual cycle, and of reliable anticipation of the brief fertile window: Fewer than 5 days; arguably – until we prove it definitively in large clinical trials – only 3 days. That is 3 days even when stress of any kind can and often will cause a delay of the fertile window as a consequence of the delay of ovulation.
The challenge is to detect the 3 days dictated by the functional lifetimes of the egg and sperm – in baseline as well as in the challenged (irregular) menstrual cycles.
We have the technology with which to do this. To explain, the menstrual cyclic profile data from a baseline subject of a pilot study (with the BBT for comparison) and then the data from three baseline subjects are shown here:
.
Very short fertile phase (aka fertile window)
detected via the cervix uteri
The short duration of the fertile phase is due to the very limited lifespan or viability of the ovulated egg (i.e., the egg released from the successfully matured dominant follicle) coupled with the pre-ovulation fertile days that are due to the functional lifespan of the sperm. The egg is thought to be fertilizable for only about 8 to 12 hours after ovulation (though some estimates have been for up to 24 hours after ovulation).
According to Speroff, Glass and Kase’s authoritative monograph on gynecologic endocrinology, “equally uncertain is knowledge of the fertilizable lifespan of human sperm. The most common estimate is 48 – 72 hours”, which means that the sperm is thought to be capable of fertilizing the egg for up to at most 3 days after being deposited in the vagina.
The sperm survive longer than the ovulated egg but only in the temporarily hospitable environment of the cervical mucus and epithelium turned hospitable just briefly before ovulation. Outside of that narrow window, the mucus is inhospitable to sperm, and prevents their and other microbes’ entry into the uterus. Allowing for the longer longevity of the sperm is the most difficult challenge for scientific family planning.
We have the definitive solution because we track the end-organ effects of all the inputs into the system. This affords reliable quantitative anticipation AND DETECTION of the ovulation event, both of which are necessary data (because of said dependence of sperm viability on the close proximity of ovulation).
The definitive solution is achieved by the unprecedented tracking of the dominant follicle maturation that varies from cycle to cycle, and that is followed by the unique short-term predictive peak, which drops into the ovulation marker nadir in the menstrual cyclic profile.
.
Details of folliculogenesis concluded with visual summary of electronic tracking of
Folliculogenesis-In-VivoTM
Folliculogenesis is a continuous process with well-defined morphologic and endocrine dynamics or timing of events. The dynamics of this process have been characterized biologically, and separated into the four intervals or stages of recruitment, selection, dominance and ovulation [G.D.Hodgen, Fertility and Sterility 38:28 1, 1983]. While in real life the timing of the last two stages is highly variable, in terms of the stereotypical menstrual cycle as used in textbooks, the timing of the stages of folliculogenesis is as follows:

The interval of recruitment begins at the end of the previous cycle, from the onset of menstrual bleeding to approximately day 5 to day 7 of the current cycle. During this interval, LH from the brain induces a factor from the ovarian theca cells that increases the blood supply and estrogen synthesis by the recruited follicles.
The term selection indicates the reduction of the recruited group of follicles down to the species-characteristic ovulatory quota, which in women and related primates is one. Selection is the culmination of recruitment on day 6 ± 1. Typically only one of the two ovaries sponsors recruitment and selection of the single dominant follicle, which is destined for ovulation.
Dominance is the interval of follicular growth that precedes ovulation after selection, and is achieved “typically” between days 8 and 12 of the stereotypical menstrual cycle. It appears that it is the one follicle most rapidly acquiring aromatase activity and LH receptors that becomes dominant, thus overcoming an ovarian inhibitory activity that suppresses the less-developed follicles of the recruited group.
This happens in the mid-follicular phase [the phase before ovulation]. Increasing quantities of estrogen are secreted by the dominant follicle and play a critical role in coordinating the development of the different parts of the reproductive tract: Estrogen priming is essential in the brain as well as in the cervical epithelium and mucus, and in the oviduct.
The Ovulona sensor readings rise with the rising estrogen and fall with increasing progesterone as has been directly determined in separate experiments in three mammalian species (bovine, porcine and human [menopausal, to allow determination as done in ovariectomized animals]).
The dominant follicle has a straightforward prerogative (as has been taught and cited in a patent): It must synchronize the entire reproductive system for ovulation, fertilization and implantation. Failing that synchronization task, conception will not be possible as in the case of the luteal phase defect, a frequent cause of failure to conceive by healthy women; or ovulation will be delayed as seen on the left in the composite image below. This is an abnormal cycle with short luteal phase of only 9 days (a known gynecological abnormality). Although two data points in this cycle were not recorded, the cyclic profile is clearly recognized, and the dominant follicle maturation peak would have been (if properly recorded) unusually high.

Ovulation: Once the dominant follicle has achieved the necessary size and adequate hormonal effects, final maturational changes within the follicle stimulate ovulation. Endocrinologically, the most prominent marker of impending ovulation is the LH surge, which anticipates ovulation within 9 to 12 hours, and which is under the control of the ovarian pacemaker that dictates the timing of these events.
At the time of the LH surge, the granulosa cells surrounding the follicle become transformed or “luteinized”. They become specialized toward synthesis and secretion of progesterone and this rapid increase in progesterone levels is responsible for inducing and coordinating several physiological changes in the reproductive system. The ovulation marker dip (or minimum) in the Ovulona cyclic profile detects one of the changes, due to the sensitivity of the epithelium and mucus in the posterior fornix region to the sex steroids.
When the follicle becomes luteinized (changes into a yellow “body” or corpus luteum), it produces progesterone (P4) in large quantities, very much larger than the quantities of produced estrogen (E2). This switch of the sex steroid predominance is also reflected in the post-ovulation follicular waves in the Ovulona cyclic profiles.
Here is a condensed pictorial summary of how the Ovulona tracks the process. Peruse the short PDF set including the presenter notes (via the sticky note icons in upper left), and focus on slide 3 (screenshot shown just below) in this PDF of 4 slides: https://biozhena.files.wordpress.com/2019/10/wealth-of-info-elucidation-silent-4-slides-animated-ed.-101519.pdf (the slides are accessible via the toggle switch icon in upper left corner of the PDF).
 Key:
Key:
R… Recruitment on days 1 to 5 ± 1 (data captured usually only after blood flow – due to hygiene concerns).
S… Selection on day 6 ± 1.
GC+TC E2up… Dominant Follicle Maturation: Granulosa and Theca Cells produced Estradiol (E2) rises, which drives the signal up; Dominant Follicle also initiates expression of LH Receptors.
GC P4up… After the appearance of LH Receptors, the preovulatory Granulosa Cells secrete Progesterone (P4), which drives the signal down. (That is also why the ovulation marker is a nadir (trough), the lowest minimum in the menstrual cyclic profile. In circulating blood, progesterone concentrations are at least an order of magnitude higher than concentrations of estradiol).
Ref.: Page 39 of 23rd Edition of Williams OBSTETRICS © 2010, 2005, 2001 by The McGraw-Hill Companies, Inc. (www.gums.ac.ir/Upload/Modules/Contents/asset39/williams23.pdf)
Observe: Post-ovulation data amplitudes are lower than in the estrogen-dominated pre-ovulation phase. The follicular waves data presumably reflect the luteal and granulosa cells’ E2 – P4 dynamic similar to that ascribed in slide 3 to the post-selection (post-point S) data that track the dominant follicle maturation.
The Ovulona™ Determining the Fertile Window
The important feature to point out about the Ovulona-generated cyclic pattern is that, while certain amplitude thresholds are constants set by the calibration of the sensor electronics, the boundary days of the fertile window (the beginning and the end of fertility, respectively) are variables that change from cycle to cycle as well as from woman to woman.
This is the important practical advantage and superiority over any prior art technique (where dynamic range varies from woman to woman): While the Ovulona dynamic range (the vertical span of measurement data) is woman- and cycle-independent, the opposite holds for the timing, which the biology causes to vary from cycle to cycle – and we monitor it and work with it.
Review three slides on how we monitor folliculogenesis in vivo with the Ovulona, which will, in due course, be transformed into a telemetric cervical ring:
The image just above is a screenshot of the third slide, which is animated and has clickable links to further information.
In conclusion, you can now appreciate the answer to the following question: Why will the folliculogenesis-in-vivo monitoring determine with certainty the extent of the fertile window whereas the prior art technologies could not?
Answer: Because only the information-rich menstrual cycle profile enables associating conceptions with cycle days (on which intercourse took place) definitively described by reference to the long-term and short-term predictive signals and the ovulation marker in the cyclic profile recorded by the Ovulona.
Folliculogenesis is not merely a process involving hormonal signals – it’s a process of integration of all neuroendocrinological inputs, which the cervix monitors. And the Ovulona monitors the cervix.
The ovulographic monitoring of folliculogenesis in vivo is believed to capture the fine-tuning effects on folliculogenesis of the direct neural control via ovarian and uterine innervation and the acute effects of local (autocrine and paracrine) modulatory actions. Those effects cannot be detected by the systemic peripheral variables such as the BBT or the urinary levels of hormones monitored by the commercially available home-use technologies.
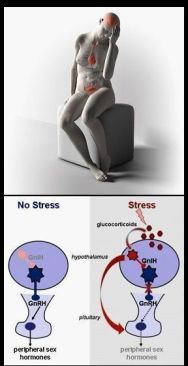
The ovulographic monitoring of folliculogenesis in vivo can determine the 3 days of the fertile window even when the timing is affected – as often happens – by stress, which is discussed in https://biozhena.wordpress.com/stress-and-fertility-fertile-window-ovulation/ .
In brief: Slow rate of descent of the Ovulona signal from the short-term predictive peak to the ovulation marker nadir (minimum) indicates an extended period of time required for the circhoral and the circamensual pacemakers (of the brain and of the ovary, respectively) to synchronize as a precondition of ovulation. (Circhoral = https://duckduckgo.com/?q=circhoral&t=newext&atb=v234-1&hps=1&ia=web AND https://www.sciencedirect.com/science/article/abs/pii/B9780122879609500320 .)
THIS REQUIREMENT OF BRAIN-OVARY SYNCHRONIZATION, THE MANDATORY PRECONDITION FOR OVULATION TO OCCUR, IS A FUNDAMENTAL PRINCIPLE.
It is speculated that an alternative synchronization activity is reflected on the descending branch of the long-term predictive peak in the record of cycle 5, which was recorded by the same woman in her menstrual cycle following after the cycle 4 with the delayed ovulation.
The information-rich (and see better in next link how) interpretable features of the menstrual cyclic profile make this possible. The star-marks in the image below highlight how the Ovulona captured the two alternative mechanisms of brain-ovary synchronization, the precondition of ovulation. (Ovulation is detected as the lowest minimum in the graph of the daily menstrual cycle readings, after the long-term and short-term predictive peaks. It is due to the switch at ovulation from estrogen predominance to progesterone predominance.)
Click on the image for better legibility in PPS.
Or click here for printable PDF of the same.
.
Let’s reiterate that folliculogenesis is a process of integration of all neuroendocrinological inputs, which the cervix monitors, and the Ovulona sensor monitors the cervix.

Folliculogenesis In Vivo™ cervix monitoring technology provides longitudinal signature records of the menstrual cycle vital sign.
For people who know women’s health, here is a single-slide pictorial synopsis with a link to a 3-page technology overview (this later edition with more pertinent links than the earlier one in the slide).