Stress And Fertility, Fertile Window, Ovulation
How stress affects the inherently narrow fertile window
Stress can do unwanted things to a woman and her menstrual cycle. In a nutshell, stress can make a woman completely infertile in the current menstrual cycle (e.g., LPD, see below), or it can delay the timing of her ovulation (and therefore the timing of the fertile window) within the menstrual cycle. Any of this can cause problems and lead to more stress…
The medical term is stress response, and it refers to the overall reaction of the organism to any adverse stimulus, whether it be of physical, mental or emotional kind, internal or external.
The purpose is to adapt to a challenge, and this goes on all the time. C’est la vie! Life is a never-ending series of stress responses.
Should the compensating reaction of the organism be inadequate or inappropriate, a pathological disorder may result.
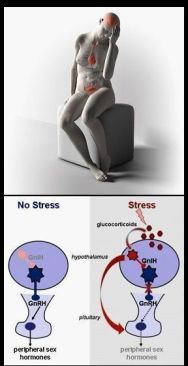
The HPA axis (the hypothalamic pituitary adrenal axis), the immune system and the central nervous system are involved in the stress response. To quote the HPA axis reference: “The HPA axis is an eloquent and ever-dynamic intertwining of the central nervous system and endocrine system.”
Pharmacological and integrative treatment of stress-induced hypothalamic amenorrhea (Genazzani A.D., Despini G., Chierchia E., Benedetti C., Prati A. (2016) Pharmacological and Integrative Treatment of Stress-Induced Hypothalamic Amenorrhea. In: Genazzani A., Tarlatzis B. (eds) Frontiers in Gynecological Endocrinology. © International Society of Gynecological Endocrinology 2016) is an example of pathological disorders the management of which will benefit from the use of our means of monitoring therapeutic interventions. Read on.
Stress and the menstrual cycle
QUOTE: “It is a matter of conventional wisdom that perturbations in the external or internal environments – that is stress – can interfere with the normal course of the menstrual cycle.” To further quote the expert, “disturbances in the menstrual cycle occur in response to exercise and physical demands, stress and emotional demands, and diet and nutritional demands” [citation below, ref. 17].
As Dr. Michel J. Ferin writes, with reference to the brain component of the female reproductive control system, “with minimal reduction in GnRH pulse frequency, small undetected defects in the follicular maturation process may occur, whereas with a higher degree of pulse inhibition the follicular phase may be prolonged, and luteal phase deficiency, anovulation, and amenorrhea may develop.”
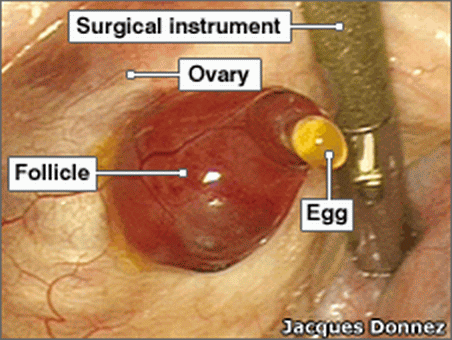
A micro-glossary: The process of follicular development of the egg (aka ovum) is also called folliculogenesis. The process takes many months, going through various stages, of which we are concerned with the final stage of the mature pre-ovulatory follicle stage. GnRH is a brain-produced hormone involved in folliculogenesis. A maturing follicle is a small, protective sac, gland, or cluster of cells in the ovary, in which an egg (ovum) develops towards ovulation (release of an ovum from the ovary), in order to have a chance to be fertilized.
The uterovaginal innervation is described in the 3-slide short PPS slide set accessed by clicking https://biozhena.files.wordpress.com/2020/08/uterovaginal-innervation-3-slides-2-pics-1-text.pps . To quote from specialist literature, it has been shown that sympathetic innervation regulates ovulation and the secretion of steroid hormones and that the sensory fibers modulate the sympathetic innervation action on ovarian functions [END OF QUOTE].
First, here is for you a baseline picture of how our folliculogenesis-in-vivoTM technique captures the course of folliculogenesis and identifies the 3 days of the fertile window in baseline subjects (defined as healthy and chemically clean i.e. taking no medication, and less than 35 years old). Take your time to study the wealth of information particularly in the right-hand part of the image (better still, use the slide linked below):
For better legibility, you can click on the image. For more detail (presented in the PDF of 4 slides best viewed – including presenter notes with further particulars – in Firefox browser; not viewed so well in Chrome and/or in AVG Secure Browser), go to: https://biozhena.files.wordpress.com/2019/10/wealth-of-info-elucidation-silent-4-slides-animated-ed.-101519.pdf .
Access the presenter notes by clicking or hovering on the sticky note icons, which the software places into the logo, seen in upper left of the PDF slides. Navigate using the down- and up-arrows in upper left of the PDF slides (in Firefox browser).
As for the scientific literature background of our work: https://biozhena.files.wordpress.com/2007/12/what-is-stress.pdf is an ad hoc selection of a few abstracts from my files in (or before) 2007, abstracts of papers addressing ovulation, reproduction, folliculogenesis and stress.
In 2007, I referred to said area of biomedical science as psychoneuroimmunoendocrinology. See if eyeballing this composite image might help you understand why.
Here is a snapshot of the clickable composite image slide, and I point out (see the fourth quadrant, lower right of the image) the importance of the new ability to correlate folliculogenesis profile data with the concurrent symptometric data. Illustrated with PMS/PMDD vs. not-PMS/PMDD diagnostic decision making, the concurrent symptometric data may prospectively be about treatment effects when managing a women’s health condition (comparing before and after treatment).
In any case: Your perusal of the selected abstracts material with my markings (highlights) will help you understand the significance of the bioZhena technology for women’s healthcare and self-care. (The footer in the document shows obsolete email and physical addresses.)
Stress and the OvulonaTM
As introduced above, our electrochemical sensor of the outer wall of the cervix, the OvulonaTM, is a smart tissue biosensor for women’s reproductive self-help. It records menstrual cycle vital sign signature data for OBGYN, PRIMARY CARE, RE and other healthcare providers’ use when needed.
Results obtained with our Ovulona prototypes lead to the conclusion that the technique appears to detect such phenomena as referred to by Dr. Ferin.
This means not merely the unprecedented tracking of different rates of dominant follicle maturation in different menstrual cycles, but even more significantly the delayed ovulations in those cycles where it takes longer than 1 day to reach the ovulation marker nadir (minimum) from the short-term predictive peak, as observed in quite a few non-baseline subjects’ cyclic profiles (in approx. 40% cycles!).
And the also-unheard-of detection of the absence of dominant follicle maturation, which makes the woman infertile in the present menstrual cycle.
Click on the composite image below for a better resolution of the contents.
Here in the upper part of the image is the detection of Ferin’s “minimal reduction in GnRH pulse frequency, small undetected defects in the follicular maturation process may occur”. The defect (delayed ovulation) is detected by the Ovulona. The delay is with respect to the hormonal signaling.
Whereas in the lower image (click for it in an animated slide with my voice), see how “with a higher degree of pulse inhibition the follicular phase may be prolonged, and luteal phase deficiency [LPD], anovulation, and amenorrhea may develop”, as writes Michel Ferin. And, indeed, we have seen the LPD, the extended follicular phase and short luteal phase, and other aberrations in the cyclic profiles of different women over the years.
bioZhena’s technique is basically detecting non-pathological stress responses in menstrual cycles through monitoring cervical end-organ effects. Pathological stress responses are captured as well.
The working hypothesis is this: The ovulographic monitoring of folliculogenesis in vivo is believed to capture the fine-tuning effects on folliculogenesis of the direct neural control via ovarian and uterine innervation.
Abnormal cyclic patterns of the end-organ effects may serve as an early warning of pathological disorders, and as a means of monitoring therapeutic interventions. This remains to be systematically investigated. Anecdotal evidence in non-baseline and in baseline cyclic profiles is compelling.
These notions are based on data from two pilot studies by independent investigators testing our prototypes at two universities. Your presenter felt compelled to seek independent test evidence to guarantee objectivity for the roots of the project, and then again to see the technique working in non-baseline subjects’ cycles tested by researchers with no interest and no involvement in the bioZhena project.
One of the studies was referred to above in the presenter notes of the second of the 4 PDF slides (the unpublished Italian baseline study by Prof. Chiara Benedetto MD PhD at Turin University, Italy) and the other study, with a number of delayed ovulations in their non-baseline subjects, is referenced and linked just below (the Marquette University Natural Family Planning Clinic study).
Do view the link above to the data from two pilot studies; presenter notes will appear upon hovering over the faint (muted) sticky note icon in upper left (click the icon for the presenter note to remain in place, click the note to minimize it into the icon).
Here is a detailed illustration (clickable for better legibility) of the detection of the frequently delayed ovulation caused by failed brain-ovary synchronization in the above depicted Hypothalamus-Pituitary-Gonad Feedback Loop.
For better legibility of the contents and for links to the references, see the PDF of the slide shown in the image: https://biozhena.files.wordpress.com/2019/01/single-slide-ovulona-detects-delayed-ovulation-w.-links.pdf . You can enlarge the contents using the browser zoom or use the PPS slide-show version of the slide: https://biozhena.files.wordpress.com/2019/01/single-slide-ovulona-detects-delayed-ovulation-w.-links.pps
There are obvious quantitative differences between the two records of the abnormal cycles with delayed ovulation. Interpretation is currently unclear even when one considers that the sensor’s signal is driven up by estrogen, and is driven down by progesterone. The sensor’s response to the two steroids was separately determined in experiments in three mammalian species (bovine, porcine and human – menopausal woman on HRT, Hormone Replacement Therapy).
There is a clear difference between the two cycles in the follicular phase data, with pronounced contrary effects on the long-term predictive peak of dominant follicle maturation: its amplitude is diminished in the long cycle vs. its amplitude is greatly enhanced in the short cycle with short luteal phase. There is also the clear difference in the extent of the luteal phases, and in the extent of the delays of ovulation. The endogenous steroids, E2 and P4, can logically be considered as being involved.
Systematic investigations should reveal the meaning of these and other deviations from baseline, and the recorded aberrations should become diagnostically meaningful and useful to women’s doctors. (And to health insurance companies: they’ll be empowered to make evidence-based decisions.)
In general, non-baseline cyclic profiles can present certain quantitative deviations from baseline characteristics: e.g., their post-ovulation (luteal) phase can be not of the normal length of about 14 days (12 to 16) as in the illustrated cycles above. In such abnormal cycles with short luteal phases (<11 days) – observed more often in older women – there is a lack of synchrony due to a mismatch between the ovarian steroids and the pituitary peptides [S.K. Smith et al., J. Reprod. Fert. 75:363, 1985].
Here is another example of a non-baseline cyclic profile of a woman with a short luteal phase (8 days); for comparison, the woman’s BBT (basal body temperature) profile in the same cycle is also shown:
A woman’s history of amenorrhea and/or of ovarian cysts is pertinent to the case of abnormally short luteal phase, but so is stress (of any kind) and its effect on the GnRH hormone generator in the hypothalamus of the brain, which affects the output of the pituitary peptides.
For example, it is known in a general way that norepinephrine and possibly epinephrine in the hypothalamus increases the GnRH pulse frequency. Conversely, the endogenous opioid peptides, the enkephalins and beta-endorphin, reduce the frequency of the GnRH pulses. These interactions are particularly important at the time of the “mid-cycle” LH surge, affecting its timing and intensity [W.F. Ganong, Review of Medical Physiology, 17th edition, Appleton & Lange, 1995, Chapter 23].
Fundamental
The slow rate of descent of the Ovulona signal from the short-term predictive peak to the ovulation marker trough (minimum) is a useful diagnostic feature that is indicative of an extended period of time required for the two “clocks” (the circhoral and the circamensual) to become synchronized as the absolute precondition of ovulation.
It is an interesting speculation that an alternative synchronization activity may be reflected in the cyclic record (on the descending branch of the long-term predictive peak in Fig. 5C) just above. This was recorded by the same woman in her menstrual cycle following after the cycle 4 with the delayed ovulation, discussed earlier (see Patient 2 in the image “Ovulona detects delayed ovulation”).
The data from those two consecutive cycles of Mrs. L.K. (cycles 4 and 5) are used in the following composite image to outline the algorithm (see the callout indications in ALGORITHM APPLIED). This enables the management of the ovulation delay phenomenon as a consequence of any kind of stress (whether it be of physical, mental or emotional kind, internal or external).
The repeatable and interpretable features of the menstrual cyclic profile make this possible, as illustrated here (click the image, and then again to enlarge and to scroll, for better legibility). The subsequent cyclic profile record (cycle 5) is shown for comparison, and an intriguing question arises (below).
An intriguing question for a future exploration of the cyclic profile: Are the two signal arrests in the descending branch of the dominant follicle maturation peak (the long-term predictive peak) due to the reproductive system effort to synchronize the brain – ovary feedback clocks? And therefore, since synchronization is thus achieved within the follicular phase, there is no ovulation delay in this cycle.
Two consecutive cycles of a non-baseline woman are suggestive of two ways of brain-ovary synchronization as precondition of ovulation. In cycle 4, 2 days of ovulation delay after the short-term predictive peak; and in cycle 5, 2 days of signal arrests on the descending branch of the dominant follicle maturation peak (the branch driven by ovarian progesterone), with no ovulation delay after the short-term predictive peak.
Concluding with take-home message about the
prerequisite of ovulation.
The ability to capture and manage the brain-ovary synchronization is why the OvulonaTM/OvulographTM combo is a breakthrough
For a summary brief OVULATION DETECTION IS A MUST BECAUSE BRAIN-OVARY PACEMAKERS SYNCHRONIZATION IS THE PREREQUISITE OF OVULATION go to https://biozhena.wordpress.com/ovulation-detection-is-a-must-because-brain-ovary-pacemakers-synchronization-is-the-prerequisite-of-ovulation/
Activation of the hypothalamus-pituitary-adrenal (HPA)-axis by physical, chemical, and psychological perturbations is known to result in elevated levels of serum corticosteroid hormones. Corticosteroids are the principal effectors in the stress response and are thought to be responsible for both adaptational and maladaptational response to perturbing situations. They have profound effects on mood and behavior, and they affect neurochemical transmission and neuroendocrine control.

Cortisol, the predominant corticosteroid in primates, is often regarded as the “stress hormone” and consequently serves as a marker of stress. Also, cortisol is a catabolic hormone that functions to mobilize stored energy. It rises under conditions of energy shortage or energy demand. Cortisol can be measured in blood, urine, and saliva.
I mentioned stress in the post on Sub-fertility (or Reduced Fertility), in the following reminder. The endocrinologist professor Brown may be quoted:
“Failing to conceive when wanted is stressful and therefore favours infertility. It should be remembered that, apart from a few conditions such as blocked fallopian tubes, absent sperm and continued anovulation, most couples will conceive eventually without help. However, the modern expectation is one of immediate results, and the main function of assisted reproduction techniques is therefore to shorten the waiting time for conception.”
To which we would add that bioZhena aims to offer a more affordable (and – at least arguably – safer) alternative to the A.R.T. approach. Besides offering to women’s healthcare providers the diagnostic technique with the capabilities outlined in the foregoing.
For an overview of examples how this technology will help women’s physicians, visit https://biozhena.files.wordpress.com/2019/01/Single-slide-How-the-Ovulona-will-help-physicians.pdf .
References as excerpted from our White Paper:
[17] Michel J. Ferin, “The menstrual cycle: An integrative view”, Chapter 6 in [2], pages 103 – 121.
[2] Eli Y. Adashi, John A. Rock, and Zev Rosenwaks, editors, “Reproductive Endocrinology, Surgery, and Technology”, Lippincott – Raven, 1996.
Terminology reminder:
Luteal phase is the phase after ovulation. Follicular phase is the phase before ovulation. Amenorrhea = abnormal absence of menstrual bleeding. GnRH = gonadotropin releasing hormone. See The Alphabet of bioZhena at /2007/11/28/the-alphabet-of-biozhena/
Terminology comment:
“Fertile window” is not a correct or precise term despite its wide use. That is because we are monitoring the woman’s biological capacity to reproduce, which is termed fecundity. Correct or precise expression is therefore “the fecund window” and “the fecund days of the menstrual cycle”.
.